3R KNOWLEDGE CENTER
i3S - Instituto de Investigação e Inovação em Saúde, Universidade do Porto
Address:
Rua Alfredo Allen, 208 4200-135 Porto, Portugal
Phone:
+351 226 074 900
Email:
3rknowledge@i3s.up.pt


By Elsa Logarinho & Ana Rita Castro, i3S
For long, animal models have been extensively explored to study physiology in order to extrapolate findings into human pathologies and therapeutical approaches. Although animal models circumvent the limitations of cellular models by providing an organismal/systemic context, it has become increasingly evident that animal models only partially reproduce human organ systems. This is particularly true for the skin and its hair follicle (HF) appendages, which architectural and cellular features are significantly different between mice (the most commonly used animal model) and humans. This has likely limited a successful translation into clinical solutions against hair loss diseases, such as androgenetic alopecia and alopecia areata. In fact, the FDA approved drugs for hair loss treatment available so far, Finasteride and Minoxidil, were identified by chance during clinical trials in which hair induction was observed as a side effect.
Ana Rita Castro et al. have recently published a review on mouse models used in hair research studies, underlining major distinctive features between mouse and human hair, discussing the limitations of the mainstream mouse models for human hair loss research, and featuring human cell-based approaches that start being prioritized.
“Ana Rita Castro, Carlos Portinha, Elsa Logarinho, The Emergent Power of Human Cellular vs Mouse Models in Translational Hair Research, Stem Cells Translational Medicine, Volume 11, Issue 10, October 2022, Pages 1021–1028, https://doi.org/10.1093/stcltm/szac059”
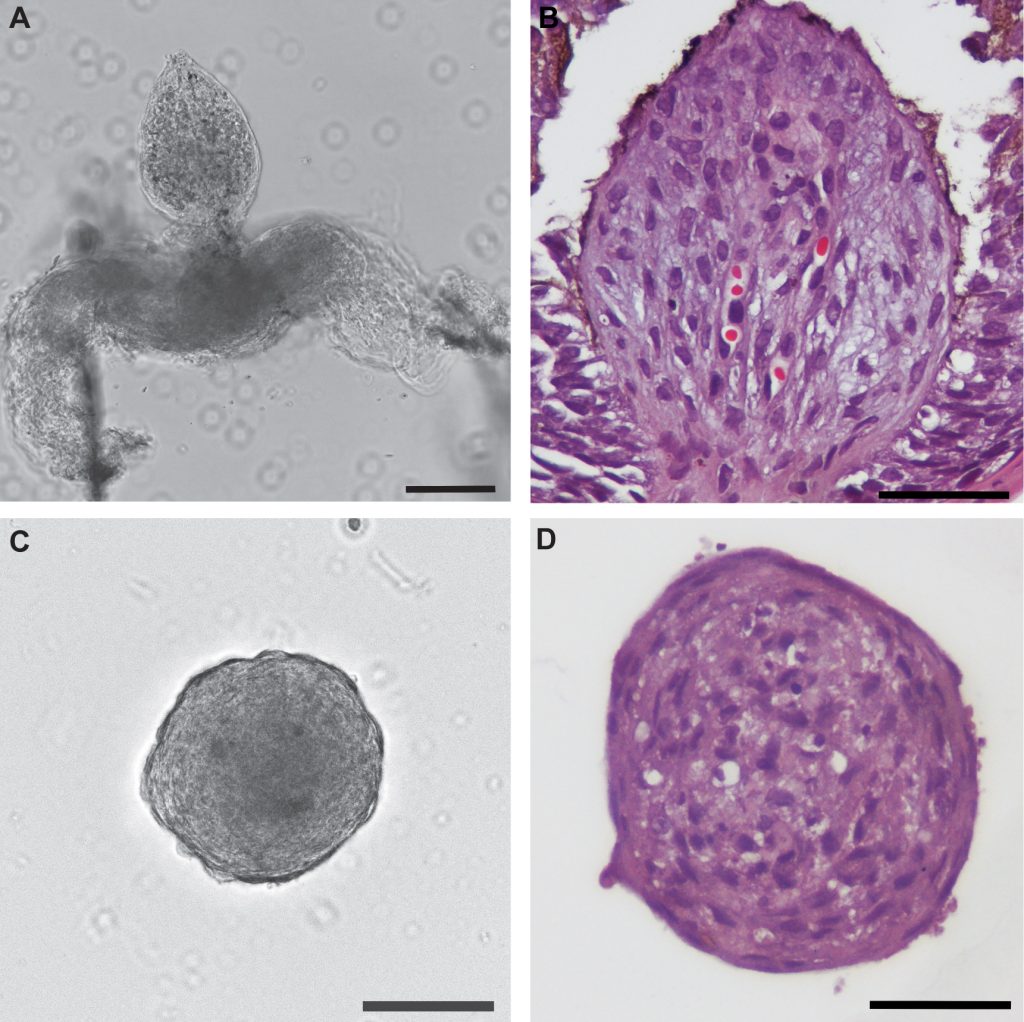
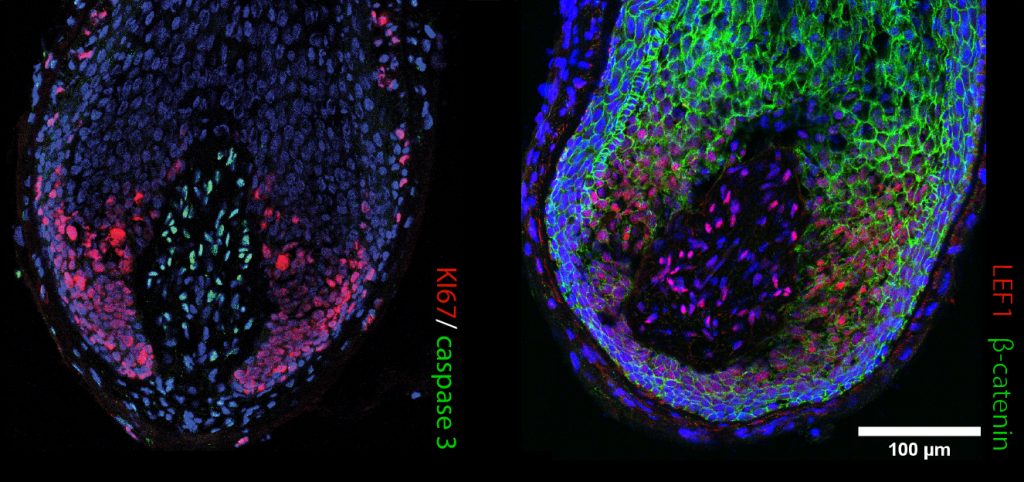
In recent years, hair researchers have developed innovative in vitro study models that better mimic human hair specificities. Besides answering the Replacement principle of the 3Rs, the non-animal-based alternative models can potentially improve clinical translation by delivering a preclinical humanized stage. Several cell-based models have emerged that more accurately forecast human hair and therapeutic response: i) in vitro 3D spheroid cultures aiming to mimic HF architecture, but still missing the physiology of the whole organ; ii) upgraded 3D co-culturing systems and bioengineered organoids, combining distinct cell types, signaling pathways and supportive scaffolds, that more closely reproduce HF function; and iii) bioprinting technology that allows large-scale production of multilayer scaffolds to construct architecturally complex organs and perform high-throughput drug screening.
Even though the human skin was one of the first organs to be bioprinted, regeneration of the hair follicle and sweat gland appendages remains intangible. However, increasingly complex bioengineered human organoid models have emerged in recent years where pigmentation, innervation, and vascularization were taken into account. For example, organoids have been developed from adult stem cells or induced pluripotent stem cells (iPSC) that are guided to differentiate and self-organize into biological functional units. Furthermore, organ-on-a-chip technology takes advantage of cultured cells/organoids and microfluidics to better recapitulate (patho)physiological responses. Finally, the sprouting artificial intelligence (AI) and machine learning revolution has resulted in robust in silico platforms to support complementary human studies. Computational models can be used for the predictive identification of druggable targets, and AI might even be applied to develop patient-tailored treatments.
In conclusion, while a single human-based in vitro/in silico model may seem insufficient, a combination of different approaches may prove robust in predicting treatment/disease outcomes of human patients and contribute to the reduction and replacement of animal experimentation. Moreover, considering the expensiveness of animal experiments, the in vitro human cell-based models may expectedly become a priority in sustainable preclinical research worldwide. Therefore, research and clinical institutions need to adopt and promote alternative human-centered approaches for basic and applied investigations. The 3Rs not only abet environmental and ethical reasons but also lessens the financial burdens of research projects.