3R KNOWLEDGE CENTER
i3S - Instituto de Investigação e Inovação em Saúde, Universidade do Porto
Address:
Rua Alfredo Allen, 208 4200-135 Porto, Portugal
Phone:
+351 226 074 900
Email:
3rknowledge@i3s.up.pt
Developing increasingly sophisticated in-vitro model systems is important to reduce the reliance on animal models in biomedical research. Many steps in the biomedical research process are already done without animals, using less complex in-vitro approaches such as traditional cell cultures. However, there are limits to how much new knowledge can be gained from such relatively simple systems. To understand disease mechanisms and develop therapies, researchers need models that recapitulate the complexity of the living body, where cells of different types form organs connected and served by blood circulation and an immune system. Research to develop non-animal models belongs to the Replacement R of the 3Rs.

At i3S, we develop non-animal models based primarily on organ-on-a-chip and organoid technology. You can find out more about some of this research here.
Organ-on-a-chip systems use microfluidics technology to mimic the human physiology of a specific organ. The possibility of incorporating cells from patients makes these systems very promising for both disease modelling and personalized medicine. However, setting up the systems is often expensive and time-consuming. Research at i3S led by Daniel Ferreira has established a method through which commercially available material can be used to establish organ-on-a-chip systems in a modular way that is both cheaper and faster. This work received the International 3Rs Prize in 2022, awarded by the NC3Rs. Recently, the same authors used this technology to engineer a fully functional stomach-on-a-chip platform mimicking the architecture of the gastric mucosa and displaying peristalsis-like motion and intraluminal flow. Complex models such as this one, are a key asset in developing tools that can be used to study human physiology in a biorelevant context while helping to reduce the burden of animal experimentation.
Organ-on-a-chip technology is also the basis for Estrela Neto’s work to establish a replacement for research into bone metastasis pain. The aim is a model that mimics the sensation and perception of pain in bone metastases that will make it possible to unravel factors that initiate the process of pain in the periphery, test new drugs, and predict their efficiency. In the case of cancer research and more specifically bone pain, animal models continue to be widely used, which causes them a lot of discomfort. This model is an important alternative to significantly reduce and, ideally, replace animal tests in this type of investigation.
Since 2020, research in the REMODEL project has focused on implementing and developing organoid models. When using tissue samples from mice, these models contribute primarily to reduction (the tissue from one single mouse can generate a large number of organoids); when using patient samples organoids replace animal use altogether. Organoids available at i3S include intestinal, colon, gastric, lung, and kidney. In addition, tumoroids (organoids made out of cancer tissue) are available from the colon, stomach, intestine, breast, or brain.
For research into tumour biology, the Nanomedicines and Translational Drug Delivery group at i3S works on developing close-to-native in vitro models as tissue modelling toolboxes for assessment of the efficacy and barrier permeability of newly developed (nano-)drugs. These in vitro models can better emulate the targeted tissue microenvironment, bioarchitecture, and complexity, thus providing a more accurate prediction of therapeutic responses in a clinical setting.

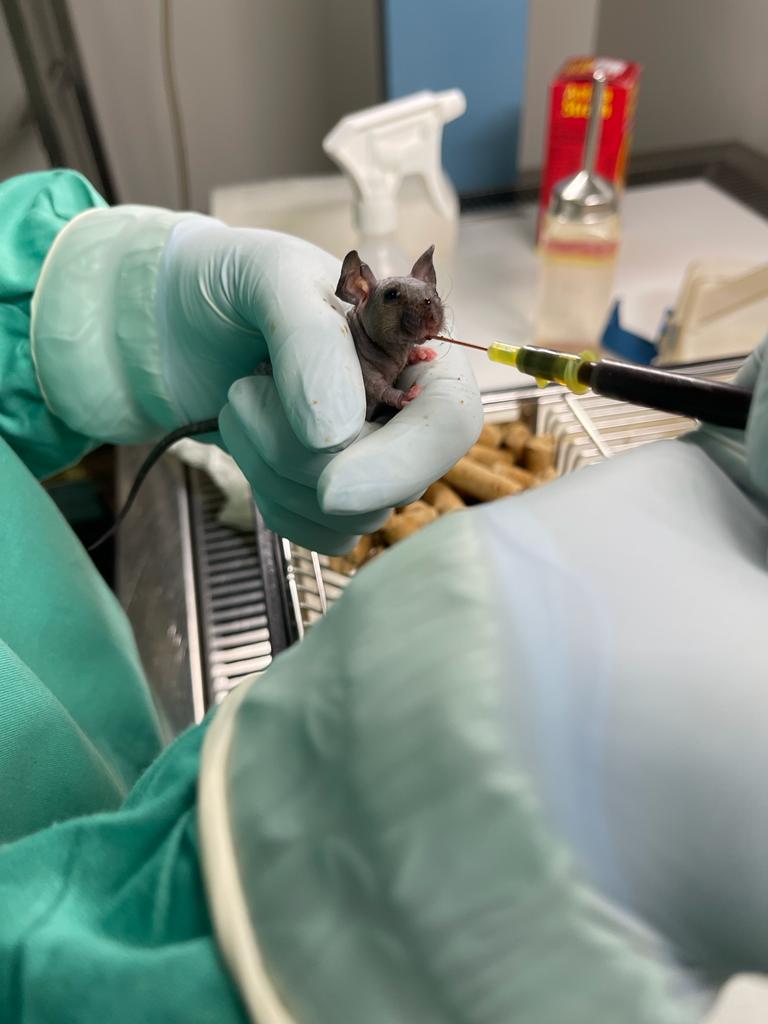
When animals are used in research, protecting their welfare as far as possible is essential. Harm should always be minimized, even when it can’t be completely avoided. Implementing the best possible care and veterinary treatment goes a long way, but research to develop new methods that cause less impact on animals is also needed. Research into methods that cause less harm to animals belongs to the Refinement R of the 3Rs.
In their recent research into the impact of distinct variants of SARS-CoV-, the Immune Regulation group, together with the Thymus Development and Function group and the Animal Facility, also focused on the animal welfare dimension when mice are used to study COVID-19 infection. For laboratory mice, this always means a severe form of the disease. Wild-type mice are not susceptible to infection, which is why genetically engineered mouse models are used to study vaccines and therapy efficiency against COVID-19. One of these models is the k18-hACE2 mouse, which expresses the human ACE2 receptor, making it susceptible to SARS-CoV-2 infection. To refine this research, the team established an accurate disease score and supportive measures r to improve the welfare of these animals and increase standardization. The disease score included respiratory, neurological, and general parameters that were very useful to follow the disease progression.
Research in the Laboratory Animal Science group at i3S addresses refinement of the way that research animals are housed and handled. The main research topics are zebrafish anaesthesia, analgesia, and euthanasia methods, early pup mortality in laboratory mouse breeding, and non-invasive methods for temperature assessment. Refinement research with zebrafish has investigated rapid cooling as a euthanasia method, and the efficacy and animal welfare aspects of different anesthesia protocols. In mouse breeding, research has shown that overlap between litters in the same cage is a risk factor for early pup mortality and that contrary to widespread belief, this mortality is not due to infanticide. Studies of different approaches to non-invasive temperature have produced a method for high throughput analysis of thermography data.